
The actual NO 3 – structure is a hybrid of the three resonance structures with an equal contribution of each resonance form.View printer-friendly version: English (PDF, DOH 331-214) | Spanish (PDF, 331-214s) Therefore, all the resonance structures are equivalent. The overall charge present on each structure is thus -1. The single-bonded O-atoms carry -1 formal charge each, while the double-bonded O-atom carries zero formal charges. In each NO 3 – resonance structure, the central N-atom carries a +1 formal charge.

Three distinct resonance structures are possible for representing NO 3 –, as shown below.ĭo all three resonance structures of NO 3 – carry the same formal charges?
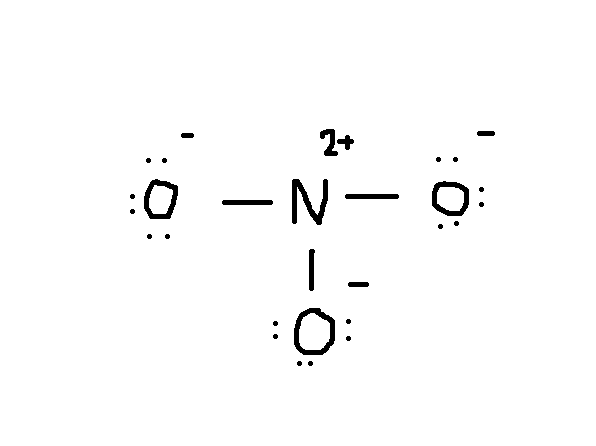
How many resonance structures are possible for representing NO 3 –? In this way, the overall formal charge on NO 3 – is -1. +1 formal charge on the central N-atom cancels with -1 formal charge of one of the two single-bonded O-atoms. What is the overall charge present on NO 3 –? No, the single bonded O-atoms carry -1 formal charge each while the double bonded O-atom carries zero formal charges. The two single-bonded O-atoms carry -1 formal charges, while the one double-bonded O-atom carries zero or no formal charges in NO 3 – Lewis structure.ĭo all three O-atoms carry the same formal charges in NO 3 –? What is the formal charge present on the O-atoms in NO 3 –? The central N-atom carries a +1 formal charge in NO 3 – Lewis structure. What is the formal charge present on the central N-atom in NO 3 –? However, two other resonance structures are possible for representing the nitrate ion, as shown below. This is the most stable Lewis structure of NO 3 – because the formal charges are as minimized in it as possible. Therefore, the NO 3 – Lewis structure is enclosed in square brackets, and a -1 formal charge is placed at the top right corner, as shown below. 1+ (-1) + (-1) = -1, which is the charge present on the nitrate ion (NO 3 –) overall in this most preferred Lewis representation. However, a +1 formal charge is present on the central N-atom, while each single-bonded O-atom carries a -1 formal charge. The above calculation shows that zero formal charges are present on the double-bonded O-atom in NO 3 – Lewis structure. ∴ The formal charge on the double-bonded O-atom in NO 3 – is 0.

The most preferred Lewis representation of NO 3 – is as shown below.

(1 single bond means 2 bonding electrons). Bonding electrons (B.E) are the total electrons shared with the atom via covalent chemical bonds.(1 lone pair means 2 nonbonding electrons). Non-bonding electrons (N.E) are the number of lone pairs present on the atom.Valence electrons can be calculated by locating the position of the elemental atom in the Periodic Table. The valence electrons (V.E) of an atom are the total number of electrons present in its valence shell.


 0 kommentar(er)
0 kommentar(er)
